Steam is a powerful force in nature, with a wide range of applications, from powering locomotives to generating electricity. It is a gaseous form of water that comes into existence when water reaches its boiling point and evaporates. At sea level, the boiling point of water is 100°C (212°F), and this is considered the minimum temperature for steam. However, steam can exist at lower temperatures under different atmospheric pressures, and its temperature can exceed 100°C when subjected to higher pressures.
What You'll Learn
- Steam is gaseous water, formed when water reaches its boiling point and evaporates
- At sea level, atmospheric pressure, the temperature of steam is 100°C
- Steam can be hotter than 100°C, depending on the pressure
- Steam can carry large amounts of thermal energy, making it desirable as a working fluid
- Superheated steam can reach temperatures of up to 600°C

Steam is gaseous water, formed when water reaches its boiling point and evaporates
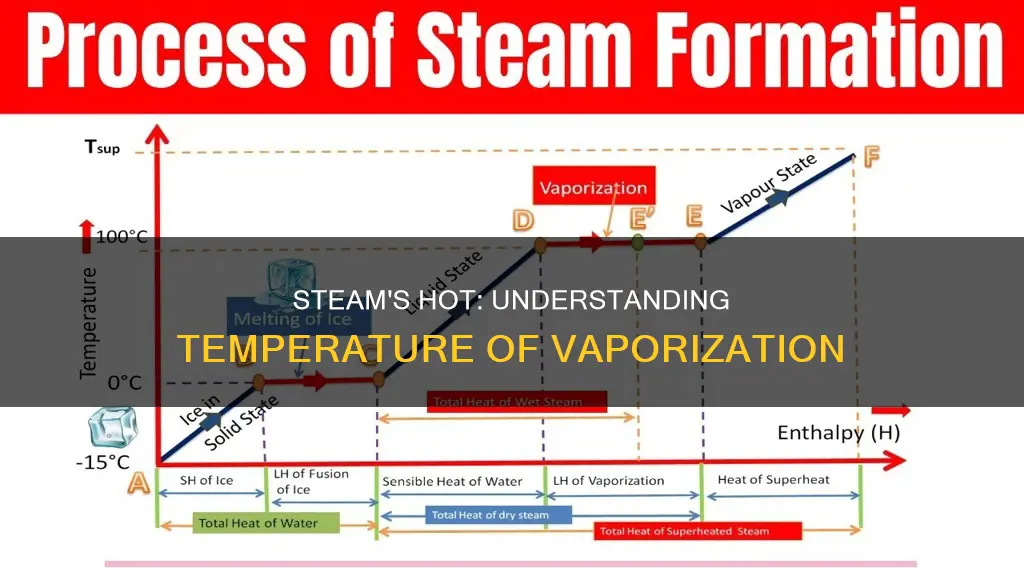
Steam is a gaseous form of water. It is formed when water reaches its boiling point and evaporates. At normal atmospheric pressure, the boiling point of water is 212°F (100°C). At this temperature, water will continue to rise in temperature until it reaches its highest boiling point. Any further increase in temperature will cause the water to evaporate into steam. This process is called boiling, and it occurs when a liquid's temperature rises beyond a specific point.
The boiling point of water is the temperature at which it changes state from a liquid to a gas. All liquids have a unique boiling point, and for water, this point is 212°F (100°C). When water is heated at normal atmospheric pressure, its temperature will increase until it reaches this boiling point. Once it reaches this temperature, any additional heat will not raise the temperature further but will instead cause the water to convert into steam. This conversion requires a significant amount of energy.
The temperature of steam can vary depending on atmospheric pressure. When the atmospheric pressure is higher than normal, more heat must be added to the water before it can turn into steam. On the other hand, when the pressure decreases, the boiling temperature of water also decreases. Therefore, steam can exist at temperatures above and below 100°C, depending on the atmospheric pressure. However, at sea level and normal atmospheric pressure, the temperature of steam is typically 100°C.
Steam has numerous practical applications. It can be used to disinfect food, sterilize equipment, and create high pressure for industrial processes. Additionally, steam is often used in power generation, such as in locomotives and electricity-generating turbines. The ability of steam to carry large amounts of thermal energy makes it a valuable working fluid in various industrial contexts.
It is important to note that exposure to steam can be hazardous. Steam can cause property damage, serious injuries, or even death if not properly controlled. Therefore, it is crucial to take the necessary precautions when working with or around steam.
Steamy Pressure Cookers: What's the Deal?
You may want to see also

At sea level, atmospheric pressure, the temperature of steam is 100°C
Steam is a powerful force in nature, capable of powering locomotives and electricity-generating turbines. It is the gaseous form of water and exists when water reaches its boiling point and then evaporates. At sea level, atmospheric pressure, the temperature of steam is 100°C (212°F). This is the highest temperature steam can reach at this pressure.
The boiling point of water is influenced by atmospheric pressure. At sea level, the atmospheric pressure is around 1013 mbar, and the boiling point of water is 100°C. As the pressure increases, so does the boiling point of water, and vice versa. Therefore, at sea level, the temperature of steam is 100°C, matching the boiling point of water at this pressure.
This temperature is crucial for various applications, such as cooking, cleaning, and sterilising equipment. It is also essential for powering machinery. Steam is an excellent working fluid due to its ability to carry large amounts of thermal energy. This property is known as the Latent Heat of Vaporization.
It is important to note that steam can be hotter than 100°C. For example, pressure cookers operate at higher pressures, resulting in steam temperatures above 100°C. Additionally, superheated steam can reach temperatures up to 600°C at atmospheric pressure.
Steaming Momos: Electric Rice Cooker Method
You may want to see also

Steam can be hotter than 100°C, depending on the pressure
Steam is the gaseous form of water and it only exists when water has been heated to its boiling point and then evaporated. At standard atmospheric pressure, the boiling point of water is 100°C (212°F), and this is also the temperature at which water transforms into steam. This transformation requires a significant amount of energy.
However, steam can indeed be hotter than 100°C. The temperature of steam is dependent on atmospheric pressure. As the pressure increases, the boiling point of water also increases, and steam generated under higher pressure can reach temperatures above 100°C. For example, a pressure cooker operates at a pressure setting of 1.8 to 2.0 bar (absolute), which is significantly higher than the boiling point of water. This increased pressure raises the temperature inside the cooker, allowing food to cook faster.
The ability of steam to carry large amounts of thermal energy is what makes it so useful in various applications, such as industrial processes and power generation. The concept of 'superheated steam' refers to steam that has been heated above 100°C, and this extra energy can be harnessed to perform tasks that require more heat energy.
At atmospheric pressure, superheated steam can reach temperatures of up to 600°C. By increasing the pressure, the boiling point of water rises, and this additional pressure requires more energy for the water to evaporate, resulting in higher temperatures. In industrial machinery, steam temperatures can range from 204°C to 538°C.
It's important to note that exposure to steam, especially at higher temperatures, can be hazardous and proper safety precautions must be taken to avoid property damage, injuries, or even death.
Steaming Caribbean Fish: A Spicy, Healthy Delight
You may want to see also

Steam can carry large amounts of thermal energy, making it desirable as a working fluid
Steam is a highly desirable working fluid due to its ability to carry large amounts of thermal energy. This property is a result of the 'Latent Heat of Vaporization'. When water is heated from 32°F to 212°F, it absorbs 180 BTUs per pound. However, converting this hot water into low-pressure steam requires an additional 970 BTUs per pound, showcasing the significant energy absorption during the phase change.
The phase change from water to steam requires a substantial amount of additional energy input. At higher pressures, even more heat must be added to water before it can turn into steam. Conversely, in a pressurized system, if hot condensate is released to a lower pressure, some of it can become steam, known as flash steam.
The enthalpy of steam, which is the sum of the enthalpy of the liquid and the enthalpy of vaporization, provides insight into its heat-carrying capacity. The high heat content of steam allows for its distribution at high pressure through small-bore pipework, making it an efficient and cost-effective medium for conveying heat energy over distances.
Steam is an excellent carrier of heat, and its use is prevalent in various industries, including food, pharmaceutical, health, petrochemical, and many more. It is also sterile, making it ideal for sterilisation in hospitals and food processing.
Furthermore, steam is easy to control due to the direct relationship between its pressure and temperature. Modern steam controls are designed to respond rapidly to process changes, and two-port control valves simplify control and installation while potentially reducing equipment costs.
In summary, steam is a desirable working fluid because it can carry large amounts of thermal energy, making it efficient and economical for heat transfer and various industrial applications. Its controllability, combined with its high heat content and ease of distribution, contribute to its widespread use across multiple sectors.
Steaming Basics: Using an Older Steamer Cooker
You may want to see also

Superheated steam can reach temperatures of up to 600°C
Steam is a powerful working fluid, largely due to its ability to carry large amounts of thermal energy. When water is heated at atmospheric pressure, its temperature rises until it reaches 212°F (100°C)—the highest temperature at which water can exist at this pressure. At this point, any additional heat does not raise the temperature but instead converts the water to steam.
The temperature of steam can be increased further by heating it at a constant pressure above atmospheric pressure. This process produces superheated steam, which has a temperature higher than its saturation temperature. Superheated steam has a wide range of applications, including in turbines for electricity generation.
The use of superheated steam in turbines also improves thermal efficiency. The steam is directed by nozzles onto a rotor, causing it to turn. The energy required for this process comes from the steam, resulting in the steam having less energy after passing through the turbine rotor. If saturated steam were used instead, the loss of energy could cause some of the steam to condense.
In summary, superheated steam is a form of steam that has been heated above its saturation temperature, and it can reach temperatures of up to 600°C. While it is valuable in certain applications, such as turbines, it is not ideal for heat transfer processes due to its lower effectiveness compared to saturated steam.
Steaming Savory: Pork and Egg Delicacy
You may want to see also
Frequently asked questions
The temperature of steam depends on the atmospheric pressure. At sea level, where the atmospheric pressure is 1013 mbar, the temperature of steam is 100°C (212°F). This is also the boiling point of water at that specific atmospheric pressure.
Yes, steam can be cooler than 100°C. Steam can exist at temperatures down to -50°C, but this is under different pressure conditions.
The maximum temperature of steam is difficult to determine, but it is likely to be in the region of 2000°C, at which point it would transition to a plasma state.







